Important clinical and epidemiological changes have been observed in lung cancer (LC) in our healthcare area compared to the previous decade. In the last 10 years, specific LC care circuits have been implemented and the active search for cases has been stepped up. The aim of this study was to analyze the progress of these changes over the last 20 years.
MethodsThis is a retrospective study comparing clinical and epidemiological changes between 2 historical cohorts of LC patients (1992–1994 [group 1, 164 patients] and 2004–2006 [group 2, 250 patients]) and a current group from the period 2011–2012 (group 3, 209 patients).
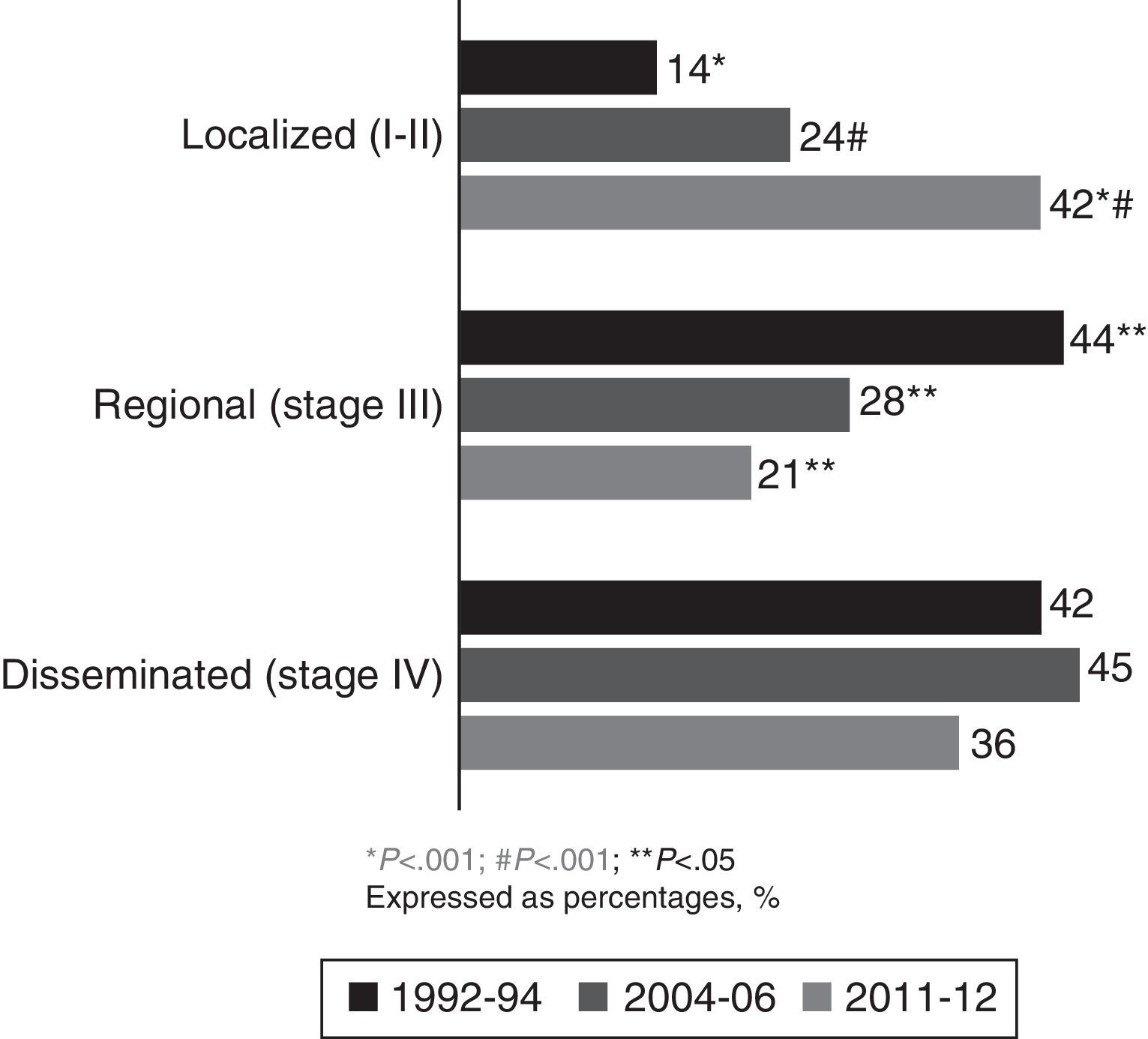
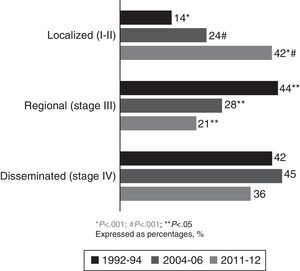
ResultsTwo hundred and nine (209) LC patients were included in group 3 (2011–2012 period). After comparing groups 3 and 2, a non-significant rise in smoking was observed in women (59% vs 41%, P=.25), while the prevalence of adenocarcinoma was unchanged (45% vs 44%, P=.9). The main changes observed were the increase in cases with previous malignancies (23% vs 16%, P=.04), the rise in patients with no associated LC symptoms (33% vs 16%, P<.001), and an increased number of localized NSCLC (non-small cell LC) diagnoses (42% vs 24% in series 2, P<.001 and 14.2% in series 1, P<.001).
ConclusionsThe number of LC patients diagnosed in localized stages has increased significantly. Furthermore, the number of patients with no symptoms associated with LC and with a history of previous malignancy was significantly increased.
En la pasada década observamos que en nuestra área sanitaria se produjeron importantes cambios clínico-epidemiológicos en el cáncer de pulmón (CP) con respecto a la década anterior. En los últimos 10 años se han puesto en marcha circuitos asistenciales específicos de CP y se ha intensificado la búsqueda activa de casos. El presente estudio fue realizado para analizar la evolución de dichos cambios 20 años después.
MetodologíaEstudio retrospectivo en el que se comparan aspectos clínico-epidemiológicos de 2 series históricas de pacientes con CP (periodo 1992-1994 [serie 1, 164 pacientes] y periodo 2004-2006 [serie 2, 250 pacientes]) con una serie actual correspondiente al periodo 2011-2012 (serie 3, 209 pacientes).
ResultadosSe incluyeron 209 pacientes del periodo 2011-2012 (serie 3). Al comparar las series 3 y 2 se observa un aumento no significativo de la frecuencia de tabaquismo en la mujer (59% vs 41%, p=0) y se mantiene la frecuencia de adenocarcinoma (45% vs 44% p=0). Los principales cambios observados fueron el incremento de casos con neoplasias previas (23% vs 16%, p=0), de pacientes sin clínica relacionada de CP (33% vs 16%, p<0) y los diagnósticos de CPNM (CP no microcítico) en estadios localizados (42% vs 24% en serie 2, p<0 y 14% en serie 1, p<0).
ConclusionesSe ha incrementado significativamente el número de pacientes diagnosticados en estadios localizados. También han aumentado los pacientes sin clínica relacionada con CP y con el antecedente de cáncer previo.
Lung cancer (LC) has the highest incidence and mortality rates in developed countries, probably due to its frequent presentation in advanced stages.1–3 It is currently the most common malignant tumor among Spanish men, and already ranks third among women.4–7 According to the Spanish National Institute of Statistics, 21,058 deaths due to LC were recorded in 2011, 3579 of them in women. The latest trends show that while mortality in men remains stable, it has been increasing in women: between 2010 and 2012, mortality in Spain due to LC increased by 12.7% in women compared to 0.2% in men.4 Recent data indicates that the 5-year survival has remained virtually unchanged in both Europe (13%) and Spain (10.7%).3 The last 20 years have seen clinical and epidemiological changes that could be explained by the steady increase in the number of women taking up smoking and improvement in diagnostic techniques.4–15 Until a few years ago, most therapeutic decisions in LC were based on the patient's functional state, TNM staging based on tumor extent (T), lymph nodes (N) and the presence of metastasis (M), and the anatomical and pathological differentiation between small cell lung cancer (SCLC) and non-small cell lung cancer (NSCLC). Since then however, major advances in the development of new imaging techniques such as fused positron emission tomography–computed tomography (PET–CT), sample collection using endobronchial (EBUS) and esophageal ultrasound (EUS), and new approaches in thoracic surgery such as video-assisted thoracoscopic surgery (VATS) have improved disease staging.15 Care of LC patients has also improved with the introduction of rapid diagnostic units (RDU) and care pathways coordinated with primary care and other departments, with the active involvement of radiologists who report suspected LC cases to the RDU to facilitate patient access.16–18 To this must be added the case management role of nursing staff, which reinforces the efficacy and sustainability of these units to the clear benefit of the patient.16–18 These improvements, together with a greater index of suspicion among primary care doctors and more active follow-up of risk groups, justify analysis of the impact of these new developments on the epidemiology, symptoms and staging of LC. The aims of our study, therefore, were to analyze changes in LC stages at diagnosis, patient demographic and clinical characteristics, the radiological forms of presentation and the histological types of LC over the last two decades.
MethodologyRetrospective, observational study with an analytical component, comparing two historical cohorts of patients diagnosed with LC with a third cohort of patients from the current period. The first cohort consisted of 164 patients reviewed from January 1992 to December 1994 (group 1), the second group included 250 patients reviewed between 2004 and 2006 (group 2) and the current cohort, analyzed between January 2011 and December 2012 (group 3), consisted of 209 patients.
PatientsPatients were recruited on the basis of pathology reports that were conclusive for LC in patients from the Hospital Xeral (Vigo, Spain) healthcare area, a tertiary hospital with a catchment area of 350,000 inhabitants.
Patient medical records were reviewed and the following variables obtained: age, sex, smoking habits, occupational exposure, neoplastic disease, chronic obstructive pulmonary disease (COPD) and tuberculosis, as well as LC-related symptoms and type of radiological lesion at diagnosis. Tumor characteristics such as histological type and staging were also analyzed. Patients were classified by histological type into squamous cell carcinoma, adenocarcinoma, large cell carcinoma and small cell carcinoma. In the case of non-small cell carcinomas, staging was based on the TNM classification in use at any particular time: stages Ia, Ib, IIa and IIb were defined as localized, stages IIIa and IIIb as regional and stage IV as disseminated. For the purpose of this study, data from the first cohort were obtained and analyzed in 1995, those from the second cohort in 2007 and those from the third cohort in 2013.
Statistical AnalysisData were analyzed using two-sided tests; P values<.05 were considered significant. Qualitative variables were expressed as percentages and frequencies, and numerical variables as mean±standard deviation (SD). The Chi-square test and Fisher's exact test were used for statistical analysis of qualitative variables. The Student's t-test was used for comparative analysis of numerical variables if the distribution was normal, otherwise non-parametric techniques were used. Normal distribution was confirmed using the Kolmogorov–Smirnov test. The analyses were performed using the Statistical Package for Social Sciences, version 15.0 (SPSS, Chicago, IL, USA).
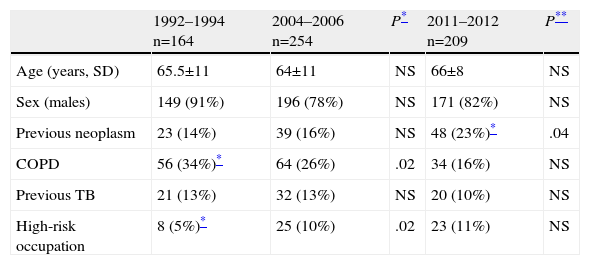
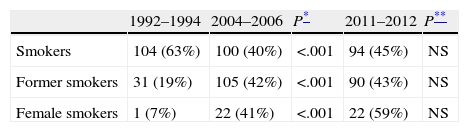
ResultsData from the current cohort of 209 patients studied between 2011 and 2012 were similar to those from previous series: 82% were male, with a mean age of 66 years (Table 1). With respect to smoking habits, 45% of patients in the last group analyzed were active smokers and 43% were former smokers. These findings are similar to those of group 2 (Table 2), maintaining the difference previously found between the two earlier cohorts. A non-significant increase in the percentage of female smokers was found, confirming the trend observed in the comparison between 1994–1996 and 2004–2006 groups (Table 2). Patient histories are shown in Table 1. The significant increase in the number of patients with a prior diagnosis of neoplasia is notable: from 14% to 23% (P=.04). The most common types of cancer observed were, in decreasing order: colorectal carcinoma, bladder carcinoma and prostate adenocarcinoma.
Personal History.
| 1992–1994 n=164 | 2004–2006 n=254 | P* | 2011–2012 n=209 | P** | |
| Age (years, SD) | 65.5±11 | 64±11 | NS | 66±8 | NS |
| Sex (males) | 149 (91%) | 196 (78%) | NS | 171 (82%) | NS |
| Previous neoplasm | 23 (14%) | 39 (16%) | NS | 48 (23%)* | .04 |
| COPD | 56 (34%)* | 64 (26%) | .02 | 34 (16%) | NS |
| Previous TB | 21 (13%) | 32 (13%) | NS | 20 (10%) | NS |
| High-risk occupation | 8 (5%)* | 25 (10%) | .02 | 23 (11%) | NS |
COPD: chronic obstructive pulmonary disease; TB: tuberculosis.
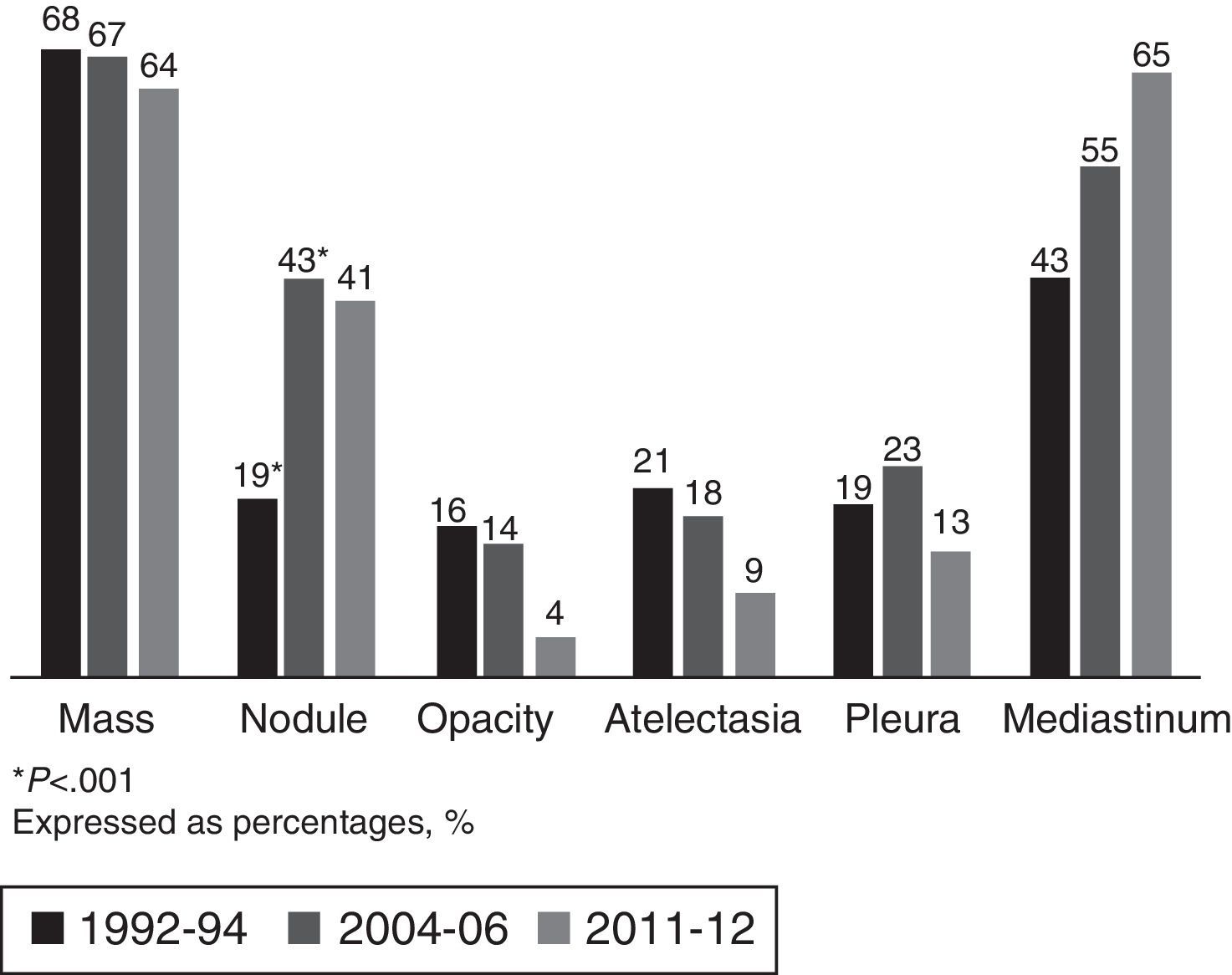
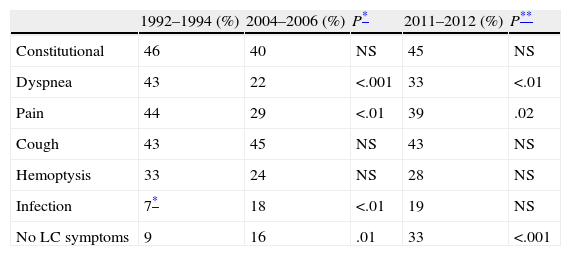
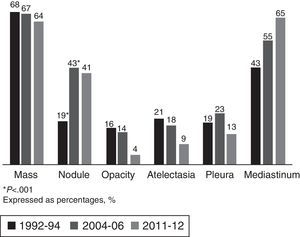
The most common LC-related symptoms at diagnosis were still cough and constitutional syndrome (Table 3). In the current cohort, the number of patients with no LC-related symptoms at diagnosis increased from 16% to 33% (P<.001). In terms of the predominant type of lesion found on the chest CT scan, in the current cohort (2011–2012), the most common forms of presentation were mediastinal involvement, followed by pulmonary mass, pulmonary nodules and pleural involvement, with no changes with respect to previous cohorts (Fig. 1).
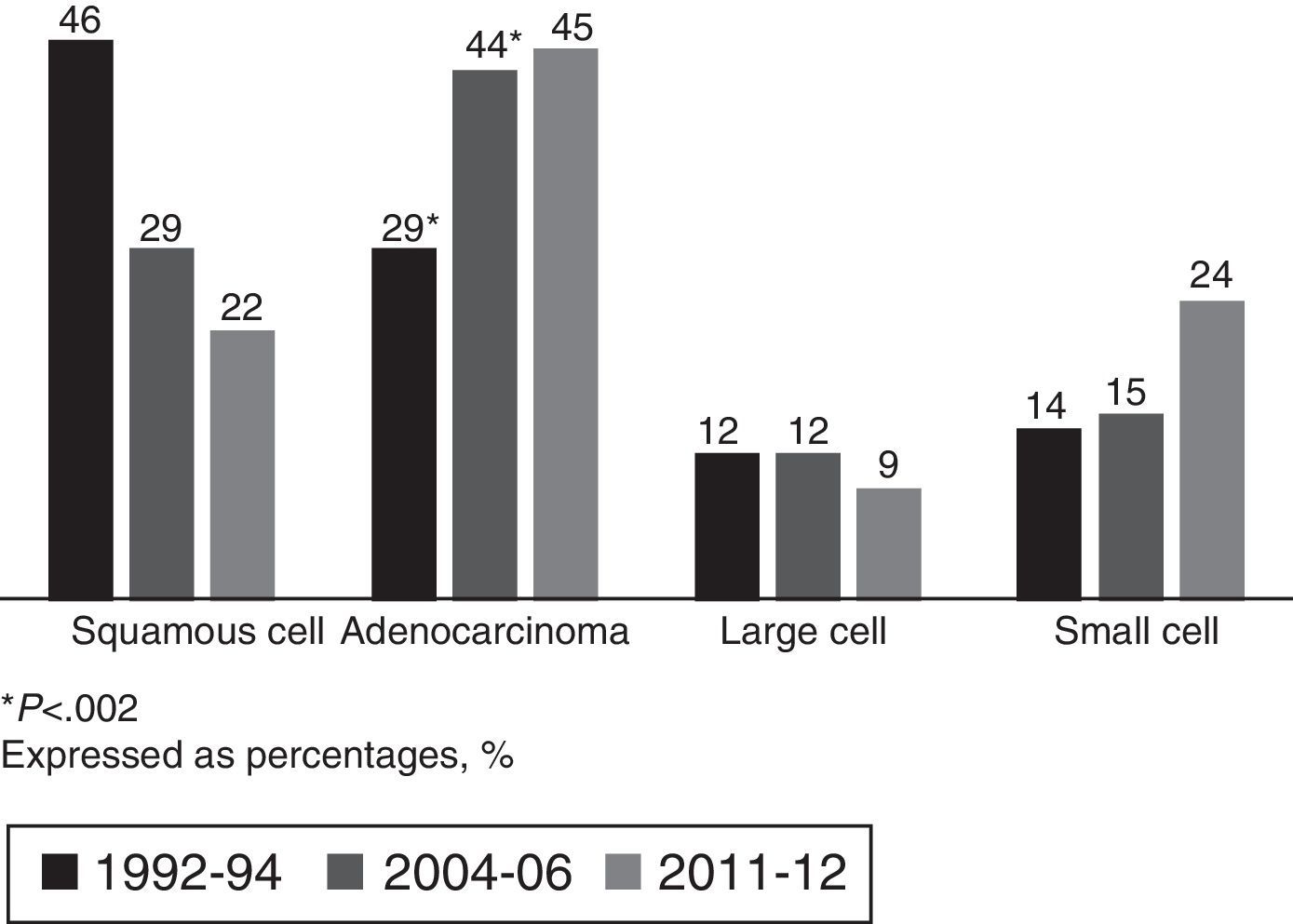
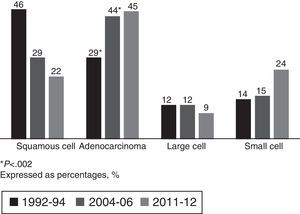
Adenocarcinoma accounted for 45% of all carcinomas in the current cohort, consolidating it as the most common histological type, with a non-significant increase with respect to the 2004–2006 group (Fig. 2).
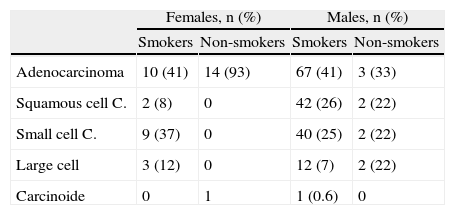
Stratified by sex and smoking habit, the most common histological type in all groups was adenocarcinoma, which is consistent with overall data. Adenocarcinoma was found in 93% in non-smoking women in the current cohort. In female smokers, although adenocarcinoma was the predominant histological type, the distribution of other types did not differ from men (Table 4).
Histological Type by Sex and Smoking Habit for 2011–2012.
| Females, n (%) | Males, n (%) | |||
| Smokers | Non-smokers | Smokers | Non-smokers | |
| Adenocarcinoma | 10 (41) | 14 (93) | 67 (41) | 3 (33) |
| Squamous cell C. | 2 (8) | 0 | 42 (26) | 2 (22) |
| Small cell C. | 9 (37) | 0 | 40 (25) | 2 (22) |
| Large cell | 3 (12) | 0 | 12 (7) | 2 (22) |
| Carcinoide | 0 | 1 | 1 (0.6) | 0 |
C., carcinoma; % of each histological type according to sex and smoking habit.
In the current cohort (2011–2012), 42% of patients had localized disease (stages Ia, Ib, IIa and IIb), at the expense of fewer diagnoses in regional and disseminated stages. Comparing current data with those from the 1992–1994 and 2004–2006 cohorts showed a significant increase in the percentage of patients diagnosed with localized NSCLC (P<.001), at the expense of a reduction in the percentage of patients with regional and disseminated disease (Fig. 3).
DiscussionComparative analysis of the characteristics of LC over two decades shows significant changes in terms of a higher percentage of patients diagnosed in localized stages, a history of previous cancer and no LC-related symptoms in their form of presentation. However, we did not find any significant differences in smoking or histological type in the last 10 years.
The significant increase in the percentage of patients diagnosed with previous malignancy has been gradual, from 14% in 2004–2006 to 23% in 2011–2012. These figures are higher than those found in other Spanish series,5–11 and could be explained by greater access to medical specialties (particularly oncology), new and better imaging techniques, and close patient follow-up. The most common previous neoplasms were colorectal and bladder carcinoma, in which smoking is also a common risk factor.19 LC has various clinical manifestations. In our 2011–2012 cohort, the most common symptoms were cough and constitutional syndrome; this has remained unchanged since the 1992–1994 group, with these symptoms presenting in more than 40% of patients in all three groups. We observed a clear increase in patients asymptomatic for LC at the time of diagnosis, double the percentage in that of previous cohorts.9 This could be due to various factors: First, greater access to imaging tests, which result in a higher number of incidental diagnoses, together with the implementation of radiological alert systems that allow suspicious findings to be centralized.18 Second, better follow-up with imaging techniques in oncology patients, who account for 23% of our sample. Third, it is interesting to note that the prevalent histological type in our area was adenocarcinoma, which tends to present as peripheral nodules that usually have later clinical manifestations.20,21 With respect to the extent of the cancer at diagnosis, it is important to highlight that in the 2011–2012 period, 42% were diagnosed in a localized stage, a significant increase with respect to previous periods, and higher than most published series.5–12 The number of diagnoses in advanced stages fell, though not as sharply as that of diagnoses in regional stages. This marked improvement in diagnoses in localized stages is consistent with the increased number of asymptomatic patients and patients with a history of malignancy. Both findings reflect the existence of improved, more easily accessible imaging techniques and better follow-up of patients with previous neoplasia or other underlying conditions. It may also be due to the introduction of care pathways that include radiological reporting systems to alert pulmonologists in RDUs. These pathways allow greater control over in- and out-of-hospital imaging services, while the creation of multidisciplinary units improves comprehensive management and improves time to diagnosis.16–18 The decrease in the number of regional stage diagnoses could also be a reflection of the improvements in mediastinal staging of LC,15 which in turn would explain the lower incidence of patients with surgical contraindications who are erroneously diagnosed with hilar and mediastinal lymph node metastatic disease using imaging tests. The correct approach to suspicion and definitive diagnosis of LC depends not only on skilled professionals, but also on good organization capable of optimizing patient coordination and ensuring their access to healthcare units, progress that has been achieved in recent years.
Given the proven causality relationship between smoking and LC, this entity would be expected to evolve in relation to smoking commencement, maintenance and cessation.12 This was reflected by the significant increase in the number of women diagnosed with LC between 1992–1994 and 2004–2006, rising from 9% to 22%, with the male/female ratio increasing from 10:1 to 4:1. This correlates with more women taking up smoking in a healthcare area that was, and is, mostly industrial.9 As expected, this trend has stabilized at present, with women accounting for 18% of LC diagnoses and only minor changes in the male/female ratio (4:1–4.5:1). Comparing the 1992–1994 and 2004–2006 cohorts in terms of histological type shows a change in trend, suggesting that adenocarcinoma has become the most common histological type to the detriment of squamous cell carcinoma. This trend was also noted in the 2011–2012 cohort, with adenocarcinoma accounting for 45% of all tumors diagnosed. This is not only consistent with the trends in two previous periods, but also with new findings in other series in Spain4–7,10 and the rest of Europe,22 Asia23 and the United States.12 In the Galician region of Spain specifically, similar results have been observed in the neighboring healthcare area of Ourense.7,13 Similarly, if we analyze the tumors according to sex, adenocarcinoma was the most common histological type in both men and women, and its frequency in smokers was independent of gender (41%). It accounted for 93% of diagnoses in female non-smokers, although there were only 15 patients in this group in our series. The higher prevalence of adenocarcinoma appears to be related to several factors: First, to changes in the characteristics of cigarettes (filtered and low in nicotine).19,24 Second, to changes in adenocarcinoma classification criteria,20,21 which now include tumors previously classified as bronchioloalveolar carcinoma. Third, to the increase in the number of incidental diagnoses, and finally, to the number of women diagnosed. It is known that female gender is associated with adenocarcinoma due to genetic and hormonal factors, the higher incidence of passive smoking and the higher frequency of epidermal growth factor receptor (EGFR) mutation in female non-smokers.14
Due to its retrospective nature based on clinical history reviews, our study has some inherent limitations. Some data may not have been collected in full, and the patient reports used were somewhat heterogeneous. In contrast, the radiology and pathology reports are objective, reliable data sources that standardize the characteristics of the three series studied. Prospective, multicenter studies in our region would confirm these findings. Patients referred to our hospital by other centers, mainly to complete diagnostic study and/or staging using EBUS and to undergo chest surgery, were eliminated from our study in order to minimize bias toward patients who, a priori, presented in a localized stage.
In conclusion, accepting the aforementioned limitations inherent to our study methodology, we observed changes in the clinical and epidemiological characteristics of LC in our healthcare area. Adenocarcinoma was consolidated as the most common histological type. The main changes were the increase in the number of asymptomatic patients at the time of diagnosis, and the significant increase in the number of patients diagnosed in localized stages. These findings open new doors for optimizing our diagnostic–therapeutic pathways, thereby improving survival.
FundingEU Seventh Framework Program (FP7/REGPOT-2012-2013-1; Code: No. 316265, BIOCAPS).
Conflict of InterestsThe authors declare that they have no conflict of interests.
We would like to thank Dr. Antoni Tardio-Baiges (Pathologist) for his collaboration.
Please cite this article as: Leiro-Fernández V, Mouronte-Roibás C, Ramos-Hernández C, Botana-Rial M, González-Piñeiro A, García-Rodríguez E, et al. Cambios en el estadio y presentación clínica del cáncer de pulmón a lo largo de dos décadas. Arch Bronconeumol. 2014;50:417–421.